
About
The COVID-19 pandemic has highlighted the importance of rapid and accurate viral testing at the point of care to both guide patient care and support surveillance. AIM-Viral is an ancillary study of the Canadian Adaptive Platform Trial of Treatments for COVID in Community Settings (CanTreatCOVID). The specific focus of this ancillary study is to integrate a point-of-care multi-pathogen testing system into primary care clinics and emergency departments. This study will assess the acceptability and feasibility of the system and evaluate its impact on patient care, provider satisfaction, and antibiotic use.
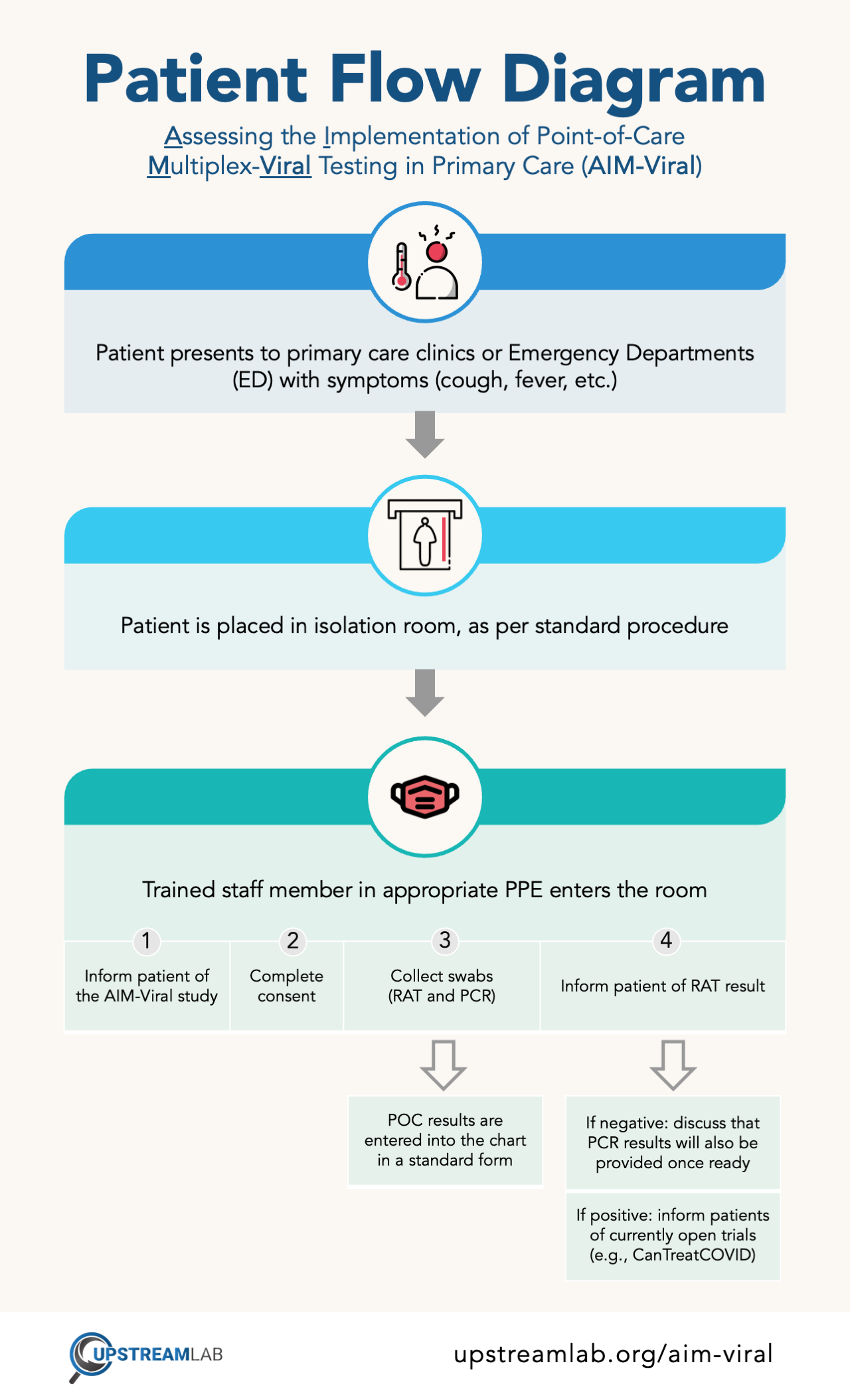
AIM-Viral will be conducted in a select number of primary care clinics and emergency departments that are part of CanTreatCOVID. After obtaining consent from patients presenting with acute upper respiratory tract symptoms, trained healthcare providers will collect nasopharyngeal swab samples. These samples will undergo rapid antigen tests for SARS-CoV-2, adenovirus, influenza A/B, and respiratory syncytial virus (RSV), as well as polymerase chain reaction (PCR) assays. This study will evaluate acceptability and feasibility through data coming from a review of electronic medical records, provider surveys, laboratory assessments, and focus groups with healthcare providers.
Impact
Patients infected with existing and novel pathogens are most likely to present first to primary care clinics and emergency departments, but access to the latest multi-pathogen point-of-care tests is limited. The real-world implications of the ability to rapidly diagnose and enroll patients into trials in these settings are substantial, particularly for investigations focusing on treatments for acute infections, where initiation of treatment is time-sensitive. The ability to promptly diagnose and enroll patients into clinical trials for acute infections could substantially expedite research processes, lead to quicker validation of therapeutic strategies, and ultimately improve patient outcomes.