
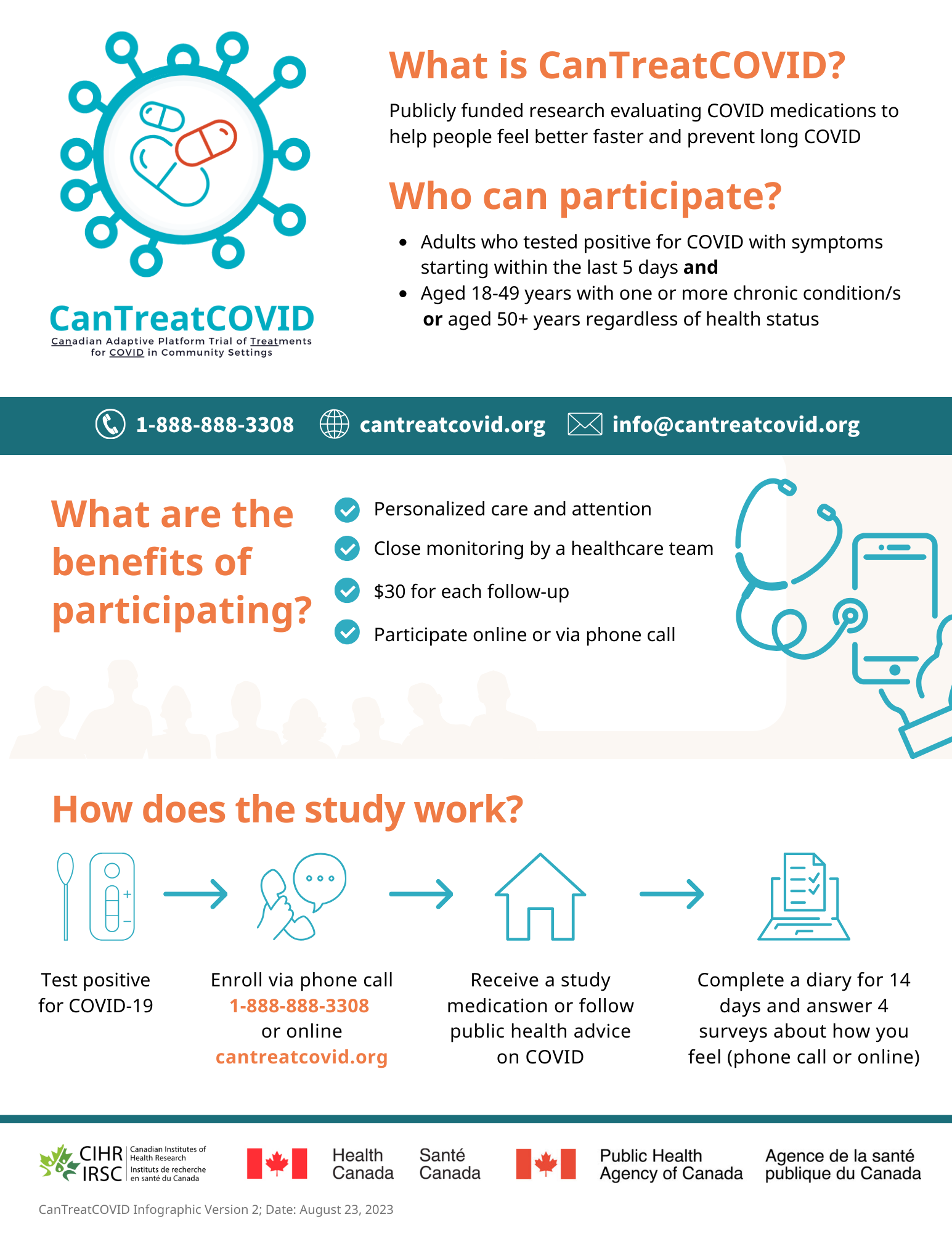
About
While public health measures and vaccines have reduced the spread of SARS-CoV-2, most scientists predict this virus will become endemic and new variants will continue to emerge. As a result, effective and affordable medications in community settings are needed.
Adaptive platform trials (APTs) are designed to compare multiple therapies and study new medications as they emerge. CanTreatCOVID will evaluate the clinical and cost-effectiveness of oral medications for SARS-CoV-2 in non-hospitalized patients. In collaboration with patient and community partners, we will engage diverse populations. The therapeutics to be investigated will be decided through a transparent Canadian COVID-19 Therapeutics Advisory Panel. CanTreatCOVID will closely work with adaptive platform trials in the United Kingdom and European Union to enable the federation of our trial data to support faster and more powerful statistical analyses.
We will measure the drug efficacy by comparing hospitalization at 28 days, as well as time to recovery and impact on “long COVID”. We will also assess changes in quality of life and health resource utilization to evaluate the cost-effectiveness of each therapeutic.
We will use numerous approaches to recruit, including a multi-faceted public communication strategy and outreach through primary care, outpatient clinics, and emergency departments. A unique strength is additional prospective recruitment using Electronic Medical Record (EMR) data from primary care research networks in Ontario, Quebec, Alberta, BC, Manitoba, and Newfoundland. Can-Treat COVID will result in a national APT infrastructure with applications beyond the COVID-19 pandemic, building capacity to efficiently study therapeutics for influenza, other upper respiratory conditions, and other emerging diseases.
To stay updated about the project, get involved, or learn about opportunities related to the project, visit the project website: CanTreatCOVID
Impact
Our findings will address some urgent public health needs and questions:
- The results of this study will help Canada and other countries in deciding whether nirmatrelvir/ritonavir is a good investment compared to fluvoxamine, and add to our current knowledge on therapeutics during the COVID-19 pandemic. Our findings will directly influence standards of care for SARS-CoV-2 infection in community settings in Canada and around the world.
- Our national APT infrastructure will be established to evaluate the effectiveness, safety, and cost-effectiveness of new therapeutics.
- Beyond SARS-CoV-2, the APT infrastructure can be adapted to study therapeutics for influenza and other upper respiratory pathogens, and for other diseases in the future.
Recruitment
Contact the study team
Toll-free line: 1-888-888-3308
Email: info@cantreatcovid.org
Website: https://cantreatcovid.org/contact/
In The Media
- What a rising summer wave says about Canada’s long-term future with COVID
- Who needs Paxlovid now? New guidelines suggest only highest-risk groups should get COVID drug
- Canada enters 5th year of COVID-19. Are we falling short in treatment?
- An ER doctor’s experience with long COVID – “My symptoms seemed endless”
- How healthcare systems are preparing for Disease X
- Is it COVID, a cold, the flu or allergies? How to tell symptoms apart — and how to stay safe
- St. Michael’s researcher secures $10 million in funding to study COVID-19 treatments
- Infectious Disease Physician Seeks COVID Patients for New CanTreatCOVID Trial
- NOSM University researchers involved in COVID-19 medication study